ALPHANATE is manufactured according to the highest quality and safety standards
A continued focus on quality and safety during the ALPHANATE manufacturing process
Plasma entering a Grifols manufacturing facility is subjected to careful controls that protect its quality through a multiphased production process. During this time, fractionation takes place. The factors then go through 2 more treatments specifically designed with the capacity to inactivate or eliminate pathogens.

The intricacies of the ALPHANATE manufacturing process
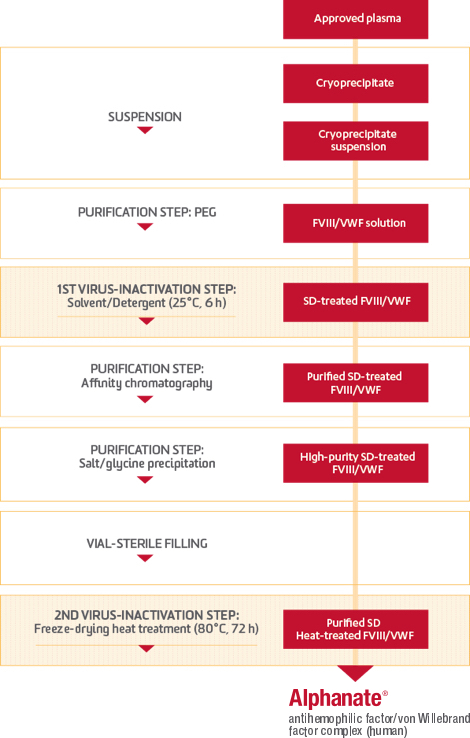
ALPHANATE is the only FVIII/VWF complex manufactured with heparin affinity chromatography
Dual-affinity chromatography
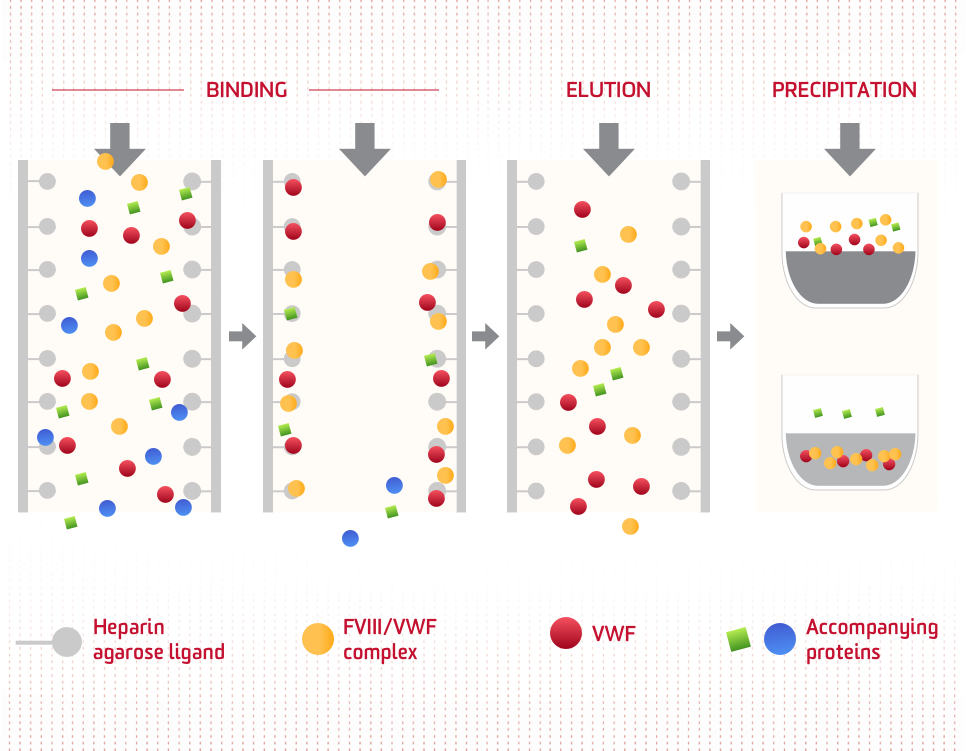
Selective isolation in support of high-purity factor concentrate: Heparin affinity chromatography specifically binds to the FVIII/VWF complex. Most of the other accompanying proteins do not bind or are removed following NaCl precipitation.
The manufacturing process for ALPHANATE demonstrates the capacity to eliminate and inactivate a wide panel of viruses
Total virus-reduction capacity for combined elimination steps1
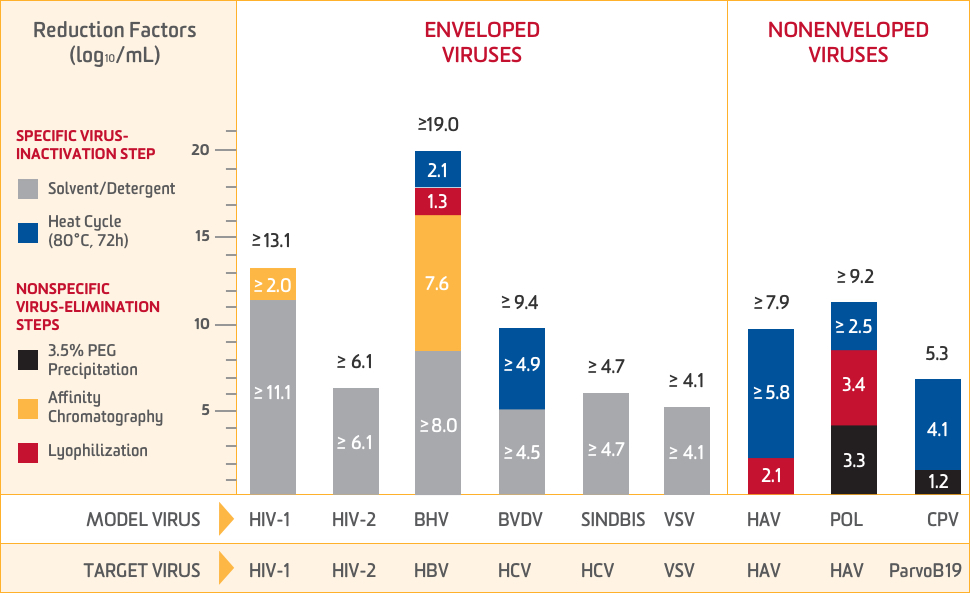
Model viruses tested1
| HIV-1 Human immunodeficiency virus-type 1, single-stranded RNA, enveloped | BHV Bovine herpesvirus, double-stranded DNA, enveloped |
| HIV-2 Human immunodeficiency virus-type 2, single-stranded RNA, enveloped | POL DNA polymerase, single-stranded RNA, nonenveloped |
| VSV Vesicular stomatitis virus, single-stranded RNA, enveloped | HAV Hepatitis A virus, single-stranded RNA, nonenveloped |
| SINDBIS Sindbis virus, single-stranded RNA, enveloped | CPV Canine parvovirus, single-stranded DNA, nonenveloped |
| BVDV Bovine viral diarrhea virus, single-stranded RNA, enveloped |
ALPHANATE has FDA-approved labeling for prion removal
The manufacturing process of ALPHANATE has the capacity to decrease the infectivity of models for the variant Creutzfeldt-Jakob disease (vCJD) and CJD agents if they were present in source material.
Because ALPHANATE is made from human plasma, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent, despite steps designed to reduce this risk.
The manufacturing process for ALPHANATE was investigated for its capacity to decrease infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the CJD and vCJD agents. TSE reduction steps include: 3.5% polyethylene glycol precipitation, affinity chromatography, and saline precipitation. These studies provide reasonable assurance that low levels of vCJD and CJD agent infectivity, if present in the starting material, would be removed.
Prion-reduction factors
| Western blot* reduction capacity (log10) | Bioassay reduction capacity (log10) | |
|---|---|---|
| PEG precipitation | 3.32 | 3.23 |
| Affinity chromatography | ≥3.46 | 3.50 |
| Saline precipitation† | 2.28 | 1.36 |
| PEG precipitation | |
| Western blot* reduction capacity (log10) | 3.32 |
| Bioassay reduction capacity (log10) | 3.23 |
| Affinity chromatography | |
| Western blot* reduction capacity (log10) | ≥3.46 |
| Bioassay reduction capacity (log10) | 3.50 |
| Saline precipitation† | |
| Western blot* reduction capacity (log10) | 2.28 |
| Bioassay reduction capacity (log10) | 1.36 |
*Western blot assay: representative (average) reduction factor and clearance factor for 2 types of PrPSc spikes: membrane-associated fraction and detergent-solubilized fraction. †Saline precipitation: calculated using clearance factor.
FVIII, factor VIII; VWF, von Willebrand factor.
Indication
ALPHANATE® (antihemophilic factor/von Willebrand factor complex [human]) is indicated for:
- Control and prevention of bleeding episodes and perioperative management in adult and pediatric patients with factor VIII (FVIII) deficiency due to hemophilia A.
- Surgical and/or invasive procedures in adult and pediatric patients with von Willebrand disease (VWD) in whom desmopressin (DDAVP) is either ineffective or contraindicated. It is not indicated for patients with severe VWD (type 3) undergoing major surgery.
Important Safety Information
ALPHANATE is contraindicated in patients who have manifested life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product or its components.
Anaphylaxis and severe hypersensitivity reactions are possible with ALPHANATE. Discontinue use of ALPHANATE if hypersensitivity symptoms occur, and initiate appropriate treatment.
Development of procoagulant activity-neutralizing antibodies (inhibitors) has been detected in patients receiving FVIII-containing products. Carefully monitor patients treated with AHF products for the development of FVIII inhibitors by appropriate clinical observations and laboratory tests.
Thromboembolic events have been reported with AHF/VWF complex (human) in VWD patients, especially in the setting of known risk factors.
Intravascular hemolysis may occur with infusion of large doses of AHF/VWF complex (human).
Rapid administration of a FVIII concentrate may result in vasomotor reactions.
Because ALPHANATE is made from human plasma, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent, despite steps designed to reduce this risk.
Monitor for development of FVIII and VWF inhibitors. Perform appropriate assays to determine if FVIII and/or VWF inhibitor(s) are present if bleeding is not controlled with expected dose of ALPHANATE.
The most frequent adverse drug reactions reported with ALPHANATE in >1% of infusions were pruritus, headache, back pain, paresthesia, respiratory distress, facial edema, pain, rash, and chills.
Please see full Prescribing Information for ALPHANATE.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1.800.FDA.1088.
Reference:
- ALPHANATE® (antihemophilic factor/von Willebrand factor complex [human]) Prescribing Information. Grifols.


